
Our lead drug candidate, Ruvembri, is an orally administered small molecule in development for the treatment of neuromuscular diseases. Based on results from cellular and animal studies, we believe Ruvembri stimulates biological resilience through activation of the MAS receptor and may have the potential to improve muscle function and preserve strength, mobility and respiratory capacity in various age-related and muscular wasting conditions.
Ruvembri ACTIVATES MAS RECEPTOR, A KEY FACTOR FOR MUSCLE METABOLISM
Our preclinical studies have demonstrated that Ruvembri
activates the MAS receptor in muscle cells, a key component of the
Renin-Angiotensin System (RAS). The RAS is a fundamental endocrine
system that is known to control fluid balance, blood pressure and
cardio-vascular function. The RAS is also involved in the regulation of
smooth, cardiac and skeletal muscle metabolism and plays a key role in
muscle function and mobility in disease states.
The mechanism-of-action of Ruvembri is illustrated below:
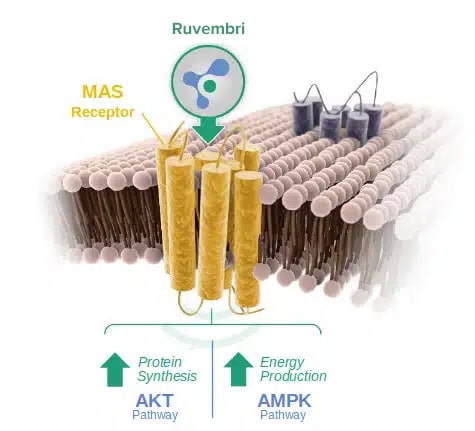
Ruvembri triggers two important
MAS receptor downstream signaling-pathways in myocytes that are impaired in many muscle wasting conditions:
- P13K/AKT/mTOR pathway – Increases protein synthesis, preserving muscle mass and increasing muscle strength
- AMPK/ACC pathway – Stimulates energy production, increasing muscle strength and mobility
MAS activation in skeletal and smooth muscles stimulates muscle metabolism and strength with a potential impact on mobility or respiratory functions.