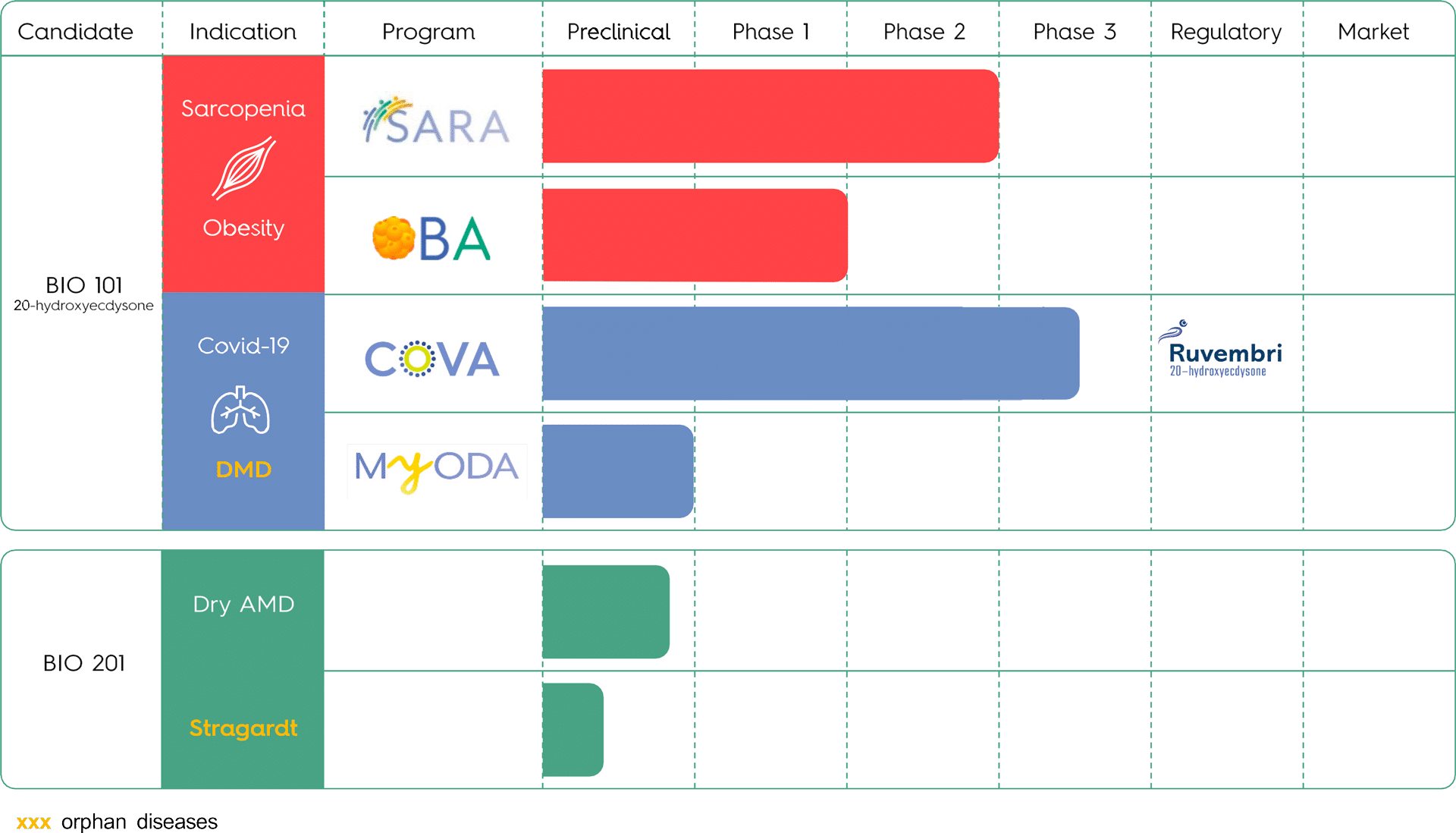
PIPELINE
SARCOPENIA (SARA CLINICAL PROGRAM)
Sarcopenia is an age-related degeneration of skeletal muscle characterized by a loss of muscle mass strength, balance and the ability to stand and/or walk. Sarcopenia leads to mobility disability in the elderly (³65 years), resulting in a loss of independence, increased risk of adverse health events, such as falls, which can shorten life expectancy.
Estimated prevalence is between 6 and 22% in the elderly (≥ 60 years), a population expected to double from approximately 962 million in 2017 to 2.1 billion by 2050.
Sarcopenia was first defined in 1989 and was classified as a disease in 2016 based on the World Health Organization (WHO) ICD-10-CM code M62.84.
There is currently no approved medication and no widely accepted standard of care for sarcopenia. Current non-medicinal treatment recommendations primarily focus on moderate physical activity and nutritional intervention.
We have tested the safety and efficacy of BIO101 (20-hydroxyecdysone) in a global, randomized, multicenter, double-blinded, placebo-controlled Phase 2 clinical trial (SARA-INT) with sarcopenic patients at risk of mobility disability. Top Line Results (TLRs) showed that BIO101 (20-hydroxyecdysone) at the highest dose (350 mg bid) produced a clinically meaningful improvement of 0.10 m/s in the 400-meter walk test (400MWT), the primary endpoint of the study. In addition, BIO101 (20-hydroxyecdysone) showed a very good safety profile at the doses of 175 mg bid and of 350 mg bid with no Serious Adverse Events (AE) related to the product. These promising results have made it possible to define the conditions for the start of a phase 3 study, never before conducted in sarcopenia.
Biophytis has obtained authorisation to launch the SARA-31 study in Belgium and the United States in the second half of 2023. The effective start of the study is planned for 2024 and will depend on the conclusion of partnership agreements and the Company’s financial resources.
OBESITY (OBA CLINICAL PROGRAM)
Worldwide, around 1 billion people live with obesity. Obesity is a chronic disease characterized by an excessive amount of body fat that disrupts the body’s normal function. Obesity treatment can lead to loss of muscle mass and function, notably because of dieting when combined with the recently introduced GLP-1 receptor agonists. Glucagon-like peptide-1 receptor agonist (GLP-1 RA) drugs are very effective drugs that lead to significant weight loss. Up to 40% of the total weight loss comes from muscle, which is a problem as muscle tissue’s role is central in controlling metabolism, on top of its motor function.
BIO101 (20-hydroxyecdysone) is the first oral daily MAS receptor activator and has demonstrated metabolic effects on muscle and fat tissues in preclinical studies in obesity. These beneficial effects of BIO101 (20-hydroxyecdysone) may translate into improved mobility and muscle strength in obese sarcopenic patients, as suggested in the SARA-INT phase 2 study. Furthermore, the 20-hydroxyecdysone molecule was already tested in obese patients during hypocaloric dieting in the Quinolia study, showing promising effects on muscle strength and fat mass loss.
With the objective of proving the efficacy of BIO101 (20-hydroxyecdysone) in the treatment for obesity in combination with GLP-1 receptor agonists, the OBA Phase 2 clinical study is expected to start mid 2024, with first patients expected to be treated in the second half of 2024. BIO101 (20-hydroxyecdysone) will be evaluated in obese patients treated with GLP-1 RAs and following hypocaloric dieting, with the first results of the efficacy of the drug candidate expected in 2025.
COVID-19 (COVA CLINICAL PROGRAM)
Approximately 20% of coronavirus 2019 (COVID-19) patients develop severe illness which may require hospitalization sometimes in intensive care unit. The mortality rate of COVID-19 mainly in elderly patients and/or with underlying comorbidities such as hypertension, cardiovascular diseases or diabetes is estimated to range between 26% and 62%. Severe illness and fatal outcomes of COVID-19 are associated with acute respiratory disease syndrome, myocardial injury, cardiac dysfunction, arrhythmias and renal alterations. Due to its mechanism of entry in human cells, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) responsible for COVID-19 notably induces an imbalance of the renin-angiotensin system (RAS) associated with excessive inflammation, apoptosis and coagulopathies.
After the pandemic phase, which had a considerable negative impact on health, economic and social levels worldwide, COVID-19 has now become an endemic respiratory viral infection on a par with influenza. Despite intensive research efforts, we are still lacking effective treatment modalities significantly avoiding mortality in patients suffering from severe forms of COVID-19.
We believe that restoration of the balance of the RAS would be a particularly relevant avenue to treat patients infected with SARS-CoV-2. Ruvembri™ activates the MAS receptor, a key component of the protective arm of the RAS, and has been shown to significantly improve respiratory function in several preclinical models. The COVA clinical program evaluates the therapeutic efficacy and the safety of Ruvembri™ as a treatment to prevent further respiratory deterioration in patients with acute respiratory failure associated with COVID-19.
The phase 2-3 COVA study, of patients 45 years and older who are hospitalized for severe respiratory symptoms and with proven COVID-19 infection, showed a statistically significant 44% reduction in the risk of respiratory failure or early death.
DUCHENNE MUSCULAR DYSTROPHY (MYODA PROGRAM)
Duchenne Muscular Dystrophy (DMD) is a rare, genetic neuromuscular disease in male children characterized by accelerated degeneration of muscles and is responsible for a loss of mobility, respiratory failure and cardiomyopathy, leading to premature death. It is the most common form of muscular dystrophy in children (first clinical symptoms are usually reported before the age of 5 years), affecting approximately one in 5,000 new born boys (approximately 20,000 new cases annually worldwide).
DMD is caused by a certain number of mutations in the dystrophin gene that results in the absence or very low levels of functional dystrophin, a cytoskeletal protein that protects muscle cells.
The absence of dystrophin in muscle severely weakens the structural and membrane stability of the muscle fibers, resulting in the loss of muscle strength and mobility, impaired respiratory function and cardiac defects. DMD evolves according to a very well understood progression with symptoms that are similar to those associated with accelerated aging across all stages.
The progression of DMD follows a highly predictable course, which is usually described in 5 consecutive stages: pre-symptomatic, early ambulatory, late ambulatory, early non-ambulatory, late non-ambulatory
Currently there is no cure for DMD and only limited treatment options primarily consisting of corticosteroids and two targeted therapies (targeting specific dystrophin mutations either by exon skipping or with stop codons) available on the market (one in the United States and one in Europe). While these treatments aim to address the root cause of DMD, by allowing a partial expression of the dystrophin gene, they do not allow full restoration of muscle cell structure as the resulting gene product is a truncated dystrophin.
In preclinical animal studies, we observed a positive effect on muscle function, mobility, and respiratory function (a major disability in later stage DMD disease progression) in mdx mice models of DMD that were treated with BIO101 (20-hydroxyecdysone).
We received orphan drug designation from the U.S. Food and Drug Administration (FDA) and European Medicines Agency (EMA) for BIO101 (20-hydroxyecdysone) in DMD in 2018. The company plans to start a Phase 1-2 clinical study (MYODA) during 2024.
DRY AGE-RELATED MACULAR DEGENERATION (MACA PROGRAM)
Age-related Macular Degeneration (AMD) is an age-related degeneration of the macula, the central part of the retina. It is one of the leading causes of irreversible vision loss and blindness in people over the age of 50 worldwide. The dry (atrophic) form of AMD affects central vision and impairs many functions affecting quality of life and independent living such as reading, driving, and facial recognition. It is a multifactorial disease that we believe is mainly caused by accumulation of A2E, a byproduct of the visual pigment cycle, that leads to retinal degeneration.
Some 85-90% of AMD patients have dry AMD in some form; either early, intermediate or late stage, known as geographic atrophy (GA). There are currently no approved treatments for any stage of dry AMD, including GA.
We are developing Macuneos (BIO201) to treat patients with intermediate dry AMD to prevent the development to advanced stages, wet AMD and GA, which lead to severe vision loss. We have completed chronic and acute animal toxicology studies required to initiate future clinical trials.